Describes Double and Triple Bonds Using Valence Bond Theory
HCN thus has one single and one triple bond. Double bonds contain a sigma bond and a pi bond.

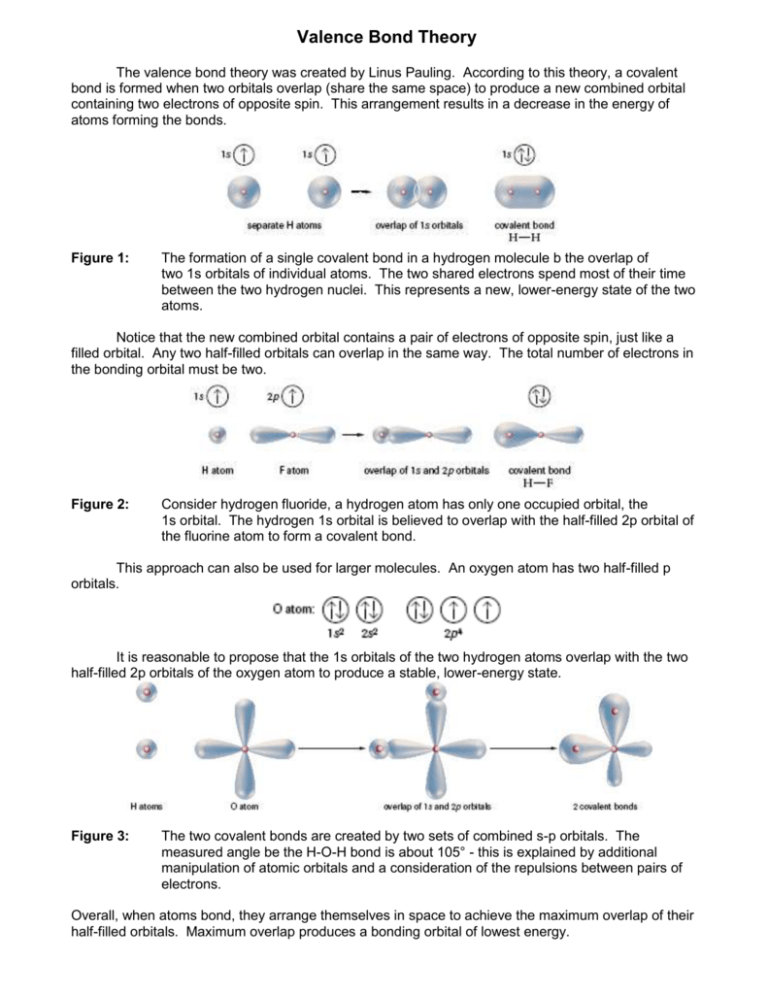
Valence Bond Theory Chemistry For Majors
According to molecular orbital theory what must be true for an interaction to be strong between two overlapping.
. They weaken the bond. Due to the overlapping electrons are localized in the bond region. They increase the distance between the bonded atoms.
What is the term for a molecular orbital that is at a. Which of the following correctly describes double and triple bonds using valence bond theory the localized electron model. O They weaken the bond.
They result from side-to-side overlap of p-orbitals. Use valence bond theory to explain why rotation is possible around single bonds but not double bonds singlesigma bonds are formed from the end to end overlap of the orbitals. They result from side-to-side overlap of p-orbitals.
Describe HCN molecular bond by using Valence Bond Theory. VALENCE BOND THEORY VB Developed by Linus Pauling two-time winner of a Nobel Prize one for Chemistry and. If waves interact destructively the resulting orbital is higher in energy.
The double bond consists of one σ bond and one π bond and the triple bond consists of one σ bond and two π bonds. They weaken the bond. Valence bond theory describes a covalent bond as the overlap of half-filled atomic orbitals each containing a single electron that yield a pair of electrons shared between the two bonded atoms.
They require the use of hybrid orbitals for all bonds. They are formed by interactions of s- and p-orbitals. They result from rotation of atoms around the bond axis.
Which of the following correctly describes double and triple bonds using valence bond theory the localized electron model. When chemical bonds form between atoms the atomic orbitals may be hybrids of sigma and pi bonds. The double bond consists of one σ bond and one π bond and the triple bond consists of one σ bond and two π bonds.
However the atomic orbitals for bonding may be hybrids. Which of the following correctly describes double and triple bonds using valence bond theory the localized electron model. Click to see full answer.
How many in each single bonds 1 sigma bond double bonds 1 sigma 1 pi triple bonds 1 sigma 2 pi b. They increase the distance between the bonded atoms. Between any two atoms the first bond formed will always be a σ bond but there can only be one σ bond in any one location.
O Triangular bipyramidal Triangular pyramidal Triangular planar Tetrahedral Which of the following correctly describes double and triple bonds using valence bond theory the localized electron model. O They result from side-to-side overlap of p-orbitals. If waves interact constructively the resulting orbital is lower in energy.
Use VB theory to describe the bonding in the AsCl 5 molecule. VALENCE BOND THEORY VBT HYBRIDIZATION. They help rotation of stoms around the bond axis.
A covalent bond forms between the two atoms by the overlap of half-filled valence atomic orbitals from each atom. Mechanism of Bonding in VB Theory. How many double bonds does HCN have.
In chemistry valence bond VB theory is one of two basic theoriesalong with molecular orbital MO theorythat use quantum mechanics to explain chemical bonding. According to VB theory a covalent bond forms from the physical overlap of half-filled valence orbitals in two atoms. Bond Order of valence e in freeunbonded atom -1.
The theory helps explain bond formation in cases where a Lewis structure cant describe real behavior. When they are rotated the overlap is broken and the bond is. They result from side-to-side overlap of p-orbitals.
Whether they occur in single double and triple bonds. Which of the following correctly describes double and triple bonds using valence bond theory the localized electron modely They increase the distance between the bonded atoms. Between any two atoms the first bond formed will always be a σ bond but there can only be one σ bond in any one location.
1Which of the following correctly describes double and triple bonds using valence bond theory the localized electron. They help rotation of atoms around the bond axis. Which of the following correctly describes double and triple bonds using valence bond theory the localized electron model.
Often the bonding atomic orbitals have a character of several possible types of orbitals. Doublepi bonds are formed from the side to side overlap of the overlap. HCN thus has one single and one triple bond.
They result from side-to-side overlap of p-orbitals. They weaken the bond. It only describes the bonding of single or double or triple bonds.
The valence-bond approach considers the overlap of the atomic orbitals AO of the participating atoms to form a chemical bond. An antibonding molecular orbital. The valence bond theory was proposed by Heitler and London to explain the formation of covalent bond quantitatively using quantum mechanics.
The greater the orbital overlap the stronger the bond. A bonding molecular orbital. In MO theory we invoke the wave nature of electrons.
Describe HCN molecular bond by using Valence Bond Theory Because px orbital of C and N will form sigma bond this leaves with two N atom p-orbitals which form two mutually perpendicular pi bonds to the two atomic p orbitals on the C atom. The regions of electron density in the bond sigma bonds have electron density along the internuclear axis bond axis pi bonds have electron density above and below the internuclear axis with a planar node along. The VB theory describes the formation of covalent.
② sp2 HYBRID ORBITALS one s. In valence bond theory the pi bond between the two oxygen atoms in O2 results from. When rotated the overlap stays the same so the bond is not broken.
Because px orbital of C and N will form sigma bond this leaves with two N atom p-orbitals which form two mutually perpendicular pi bonds to the two atomic p orbitals on the C atom. If a molecule has double or triple bonds between atoms the sigma bond is the first bond formed. In chemistry valence bond VB theory is one of two basic theoriesalong with molecular orbital MO theorythat use quantum mechanics to explain chemical bonding.
They have the same bond length as single bonds. Sp sp 2 sp 3 sp 3 d sp 3 d 2 sp 3 d 3. They result from rotation of atoms around the bond axis.
They impede rotation around the bond axis. They have the same bond length as single bonds. In terms of bond order single bonds have one sigma bond double bonds consist of one sigma bond and one pi bond and triple bonds contain one sigma bond and two pi bonds.
We say that orbitals on two different atoms overlap when a portion of one orbital and a portion of a second orbital occupy the same region of space. Triple bonds contain a sigma bond and two pi bonds. Later on Linus Pauling improved this theory by introducing the concept.

1 6 Valence Bond Theory And Hybridization Chemistry Libretexts

No comments for "Describes Double and Triple Bonds Using Valence Bond Theory"
Post a Comment